Definition: Inflammation is defined as the local response of living mammalian tissues to injury from any agent. It is a body defense reaction in order to eliminate or limit the spread of injurious agents, followed by the removal of the necrosed cells and tissues.
Causes– The injurious agents causing inflammation are:
1. Infective agents like bacteria, viruses, and their toxins, fungi, and parasites.
2. Immunological agents like cell-mediated and antigen-antibody reactions.
3. Physical agents like heat, cold, radiation, and mechanical trauma.
4. Chemical agents like organic and inorganic poisons.
5. Inert materials such as foreign bodies.
Signs of Inflammation: 4 signs of inflammation are:
i) rubor (redness);
ii) tumor (swelling);
iii) calor (heat); and
iv) dolor (pain).
To these, fifth sign functio laesa (loss of function) was later added by Virchow.
Types Of Inflammation – Depending upon the defence capacity of the host and duration of response, inflammation can be classified as acute and chronic.
A. Acute inflammation is of short duration (lasting less than 2
weeks) and represents the early body reaction, resolves quickly
and is usually followed by healing.
The main features of acute inflammation are:
1. accumulation of fluid and plasma at the affected site;
2. intravascular activation of platelets; and
3. polymorphonuclear neutrophils as inflammatory cells.
Sometimes, the acute inflammatory response may be quite severe and is termed as fulminant acute inflammation. ( for GPAT )
B. Chronic inflammation is of longer duration and occurs after delay, either after the causative agent of acute inflammation persists for a long time, or the stimulus is such that it induces chronic inflammation from the beginning.
ACUTE INFLAMMATION
The main features of acute inflammation are:
1. accumulation of fluid and plasma at the affected site.
2. intravascular activation of platelets
3. Accumulation of polymorphonuclear neutrophils as inflammatory cells at the affected site.
Acute inflammatory response by the host to any agent can be divided into two events:
I. Vascular events
II. Cellular events
The release of mediators of acute inflammation is intimately linked to these two processes.
I. VASCULAR EVENTS
Alteration in the microvasculature (arterioles, capillaries
and venules) is the earliest response to tissue injury.
These alterations include haemodynamic changes and changes in
vascular permeability.
Haemodynamic Changes
Haemodynamic Changes include the vascular flow and calibre of small blood vessels
in the injured tissue. The sequence of these changes is as under:
1. Immediate vascular response comprises of transient vasoconstriction of arterioles. With mild form of injury, the blood flow may be re-established in 3-5 seconds while with more severe injury the vasoconstriction may last for about 5 minutes.
2. Next follows persistent progressive vasodilatation which involves mainly the arterioles, but to a lesser extent, affects other components of the microcirculation like venules and capillaries.
This change occurs within half an hour of injury.
Vasodilatation results in increased blood volume, which is responsible for redness and warmth at the site of acute inflammation.
3. Progressive vasodilatation, in turn, may elevate the local
hydrostatic pressure resulting in transudation of fluid into the
extracellular space. This is responsible for swelling at the local
site of acute inflammation.
4. Slowing or stasis -Slowing or stasis of microcirculation causes increased concentration of red cells, and thus, raised blood viscosity.
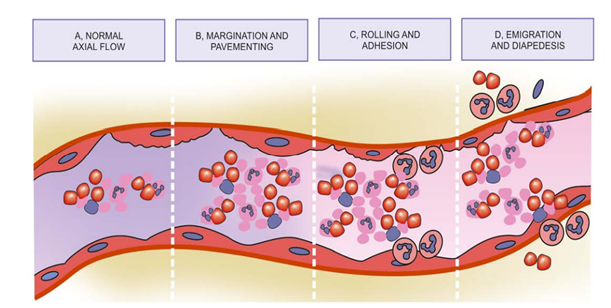
5. leucocytic margination -Stasis or slowing is followed by leucocytic margination or peripheral orientation of leucocytes (mainly neutrophils) along the vascular endothelium. The leucocytes stick to the vascular endothelium briefly, and then move and migrate through the gaps between the endothelial cells into the extravascular space. This process is known as emigration.
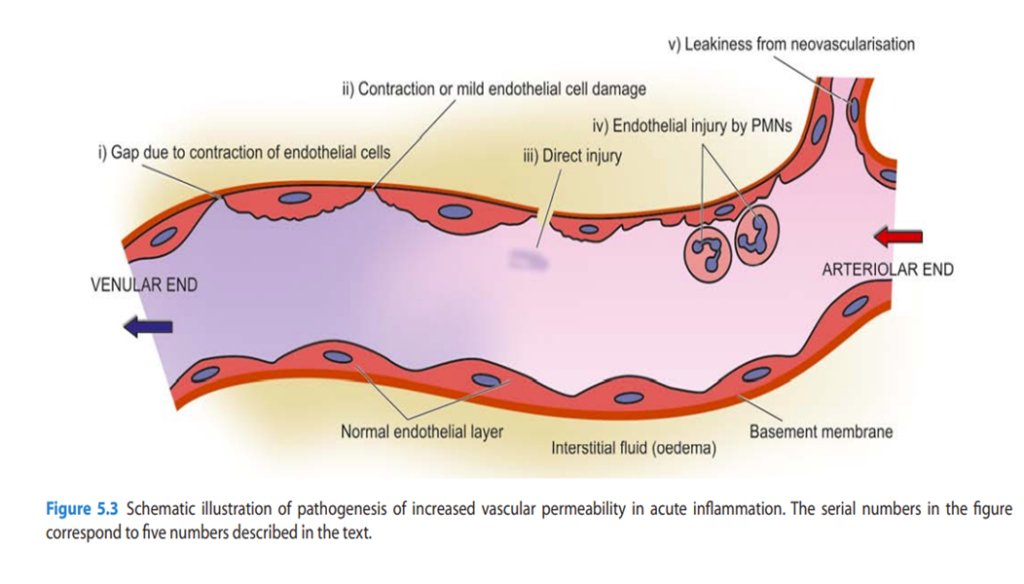
Increased Vascular Permeability
Due to increased vascular permeability in acute inflammation normally non-permeable endothelial layer of microvasculature becomes leaky
- Contraction of endothelial cells: The endothelial cells develop temporary gaps between them due to their contraction resulting in vascular leakiness. It is mediated by the release of histamine, bradykinin, and other chemical mediators.
- Contraction or mild endothelial damage: In this mechanism, structural re-organisation of the cytoskeleton of endothelial cells causes mild form of endothelial damage. This is mediated by cytokines such as interleukin-1 (IL-1) and tumour necrosis factor (TNF)- α .
- Direct injury to endothelial cells: Direct injury to the endothelium causes cell necrosis and appearance of physical gaps at the sites of detached endothelial cells. Process of thrombosis involving platelets and fibrin is initiated at the site of damaged endothelial cells.
II. CELLULAR EVENTS
The cellular phase of inflammation consists of 2 processes:
1. exudation of leucocytes
2. phagocytosis.
1. Exudation of Leucocytes
Leucocyte adhesion to endothelium– The escape of leucocytes from the lumen of microvasculature to the interstitial tissue is the most important feature of inflammatory response. Mainly, polymorpho-nuclear neutrophils (PMNs) and macrophages are involved in inflammation.
With stasis, changes in the normal axial flow of blood in the microcirculation take place. The normal axial flow consists of central stream of cells comprised by leucocytes and RBCs and peripheral cell-free layer of plasma close to vessel wall.
Due to stasis (slowing down ), the central stream of cells widens and peripheral plasma zone becomes narrower. This phenomenon is known as margination.
As a result of this, neutrophils of the central column come close to the vessel wall. this is known as pavementing.
Rolling and adhesion -Peripherally marginated and pavemented neutrophils slowly roll over the endothelial cells lining the vessel wall . This is known as rolling phase. This is followed by transient bond between the leucocytes and endothelial cells which becomes firmer. This is called adhesion phase.
The following cell adhesion molecules (CAMs) bring about rolling and adhesion phases:
- Selectins- These are a group of CAMs expressed on the surface of activated endothelial cells and are structurally composed of lectins or lectin-like protein molecules.
Their role is to recognise and bind to glycoproteins and glycolipids on the cell surface of neutrophils.
There are 3 types of selectins:
- P-selectin- Involved in rolling.
- E-selectin – Involved in both rolling and adhesion.
- L-selectin- Involved in lymphocytes to the endothelial cells in lymph nodes.
Present on the surface of lymphocytes.
- Integrins- Also help in adhesion between endothelial cells and neutrophils.
Emigration- After sticking of neutrophils to endothelium, the former move along the endothelial surface till a suitable site between the endothelial cells is found where the neutrophils throw out cytoplasmic pseudopods.
Chemokine ( protein ) act on adhering leukocyte and stimulate the cells to migrate towards the site of injury.
After exiting the circulation, leucocyte moves in the tissue towards the site of injury by a process called chemotaxis.
Phagocytosis- Phagocytosis is defined as the process of engulfment of solid particulate material by the cells (cell-eating). The cells performing this function are called phagocytes.
There are two main types of phagocytes-
- Neutrophils
- Macrophages
Phagocytosis involves the following 3 steps
1. Recognition and attachment
2. Engulfment
3. Killing and degradation
1. Recognition and attachment
Phagocytosis is initiated by the expression of cell surface receptors on macrophages which recognise microorganisms.
E.g., mannose receptor and scavenger receptor.
2. Engulfment
The microbe bound to the surface of phagocyte is ready to be engulfed.
This is accomplished by formation of cytoplasmic pseudopods around the particle due to activation of actin filaments beneath cell wall which envelopes it in a phagocytic vacuole.
3. Killing and degradation
There are different mechanisms of killing-
- Lysosomal enzymes and proteins-There are lysosomal granules present in neutrophils and macrophages which releases enzymes that damages the microbe.
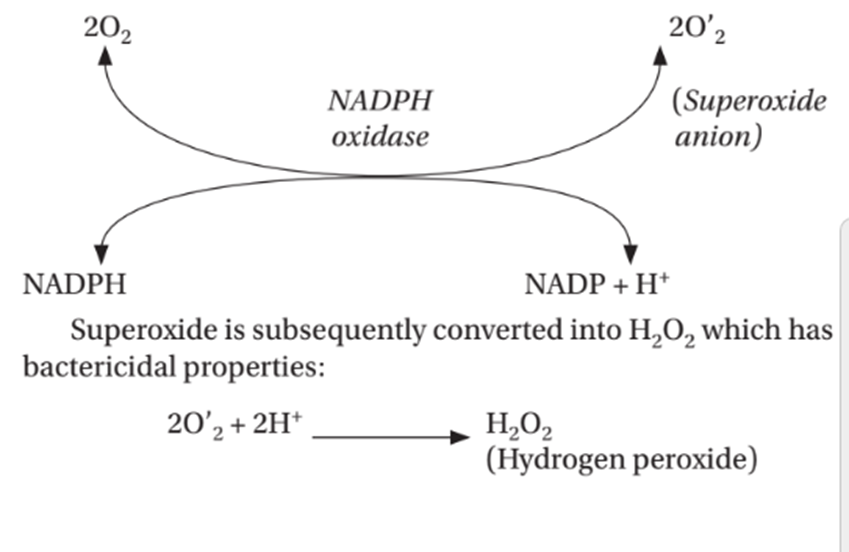
- Oxygen free radicals ( ROS ) oxidatively damage the microbes.
- These are a large and increasing number of endogenous chemical substances which mediate the process of acute inflammation.

REFERENCE
Harsh Mohan; Text book of Pathology; 6 th edition; India; Jaypee Publications; 2010